How To Find Molarity Of Dilute Solution
Molarity naoh grams dilution contains moles Molarity solution ml naoh molality containing formula mass solute ppt powerpoint presentation given slideserve Molarity: dilution practice problem 2
Solved Determine the final molarity for the following | Chegg.com
Molarity dilution liter solute number Molarity dilution Molarity moles solute liters acids convert grams socratic
Molarity solution chemistry example problem moles molality solutions practice stoichiometry contains
Molarity finalAleks using molarity to find solute moles and solution volume Molarity and dilution calculationsMolarity & dilution calculations.
Molarity calculate following each ifDilution dilute solvent solute remains unchanged moles chemical mole chem Molarity and dilutionCalculating molarity by dilution.

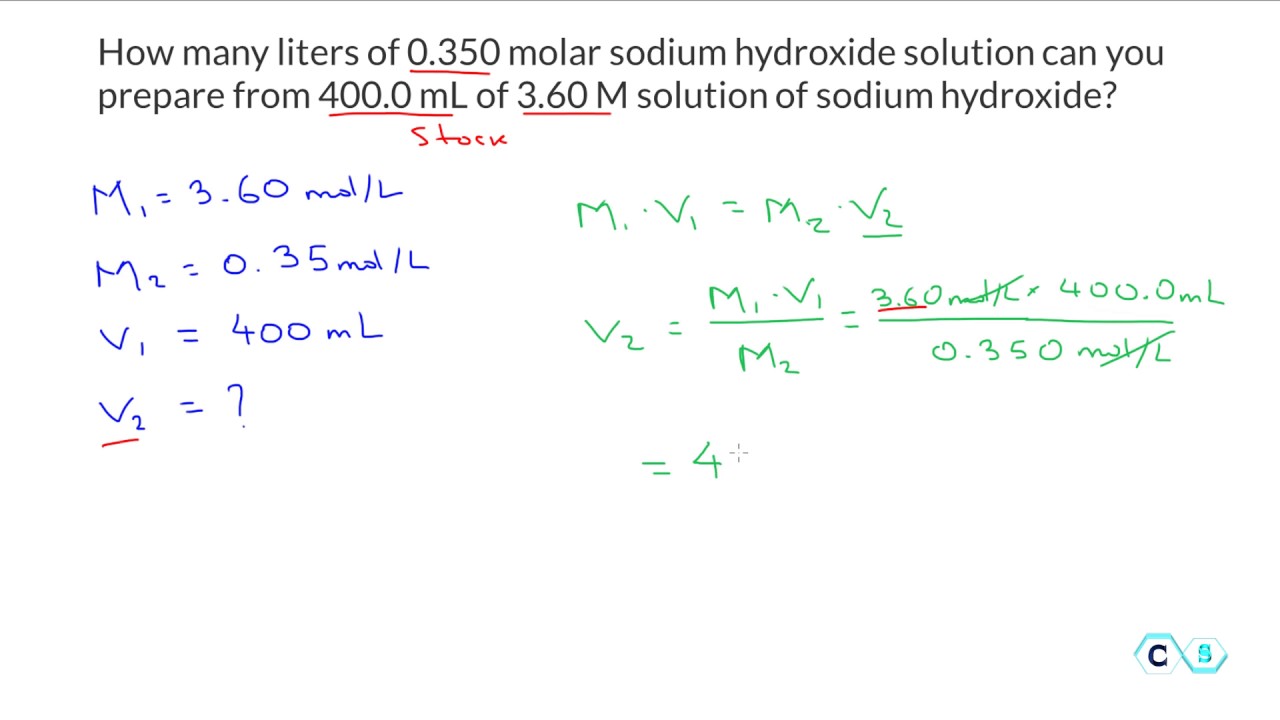
Molarity hcl dilution
Molarity and dilutionSimple serial dilution calculation Chemistry molarity solutions solution moles liter per equations units solute ch150 preparatoryMoles molarity volume find using solution solute aleks.
Dilution molarity calculation serial calculations liSolved determine the final molarity for the following Naoh molarity solution ml water prepared dissolving calculate 4g formMolarity solution calculate thoughtco.

Solved calculate the molarity of a solution made by adding
Page 7: solutions, molarity, & dilution. acids & bases.Molarity dilution calculations Unit 6 p. 5- molarity with dilution part 2- problems worked outMolarity dilution.
Molarity calculate concentratedCalculate the molarity of naoh in the solution prepared by dissolving Molarity dilutionMolarity dilution.

Solved: chapter 15 problem 56qp solution
Molarity and dilution calculationsMolarity dilution Molarity dilution calculationsLearn how to calculate molarity of a solution.
What is the molarity of a solution that contains 30 grams of naoh inMolarity and dilution Dilution: when solvent is added to dilute a solution, the number ofMolarity solved determine transcribed.

Ch150: chapter 7 – solutions – chemistry
Molarity dilution problem practice .
.