When A Solution Is Diluted The Molarity
Molarity dilution Solved a student dilutes 11.5 ml of 6 m naoh solution with Molarity solution ml naoh molality containing formula mass solute ppt powerpoint presentation given slideserve
Unit 6 p. 5- Molarity with Dilution Part 2- Problems Worked Out - YouTube
Kbr diluted homeworklib Molarity dilution Mahoney chemistry podcast 10.3 molarity and dilutions calculations
Dilution molarity calculations diluting prepared
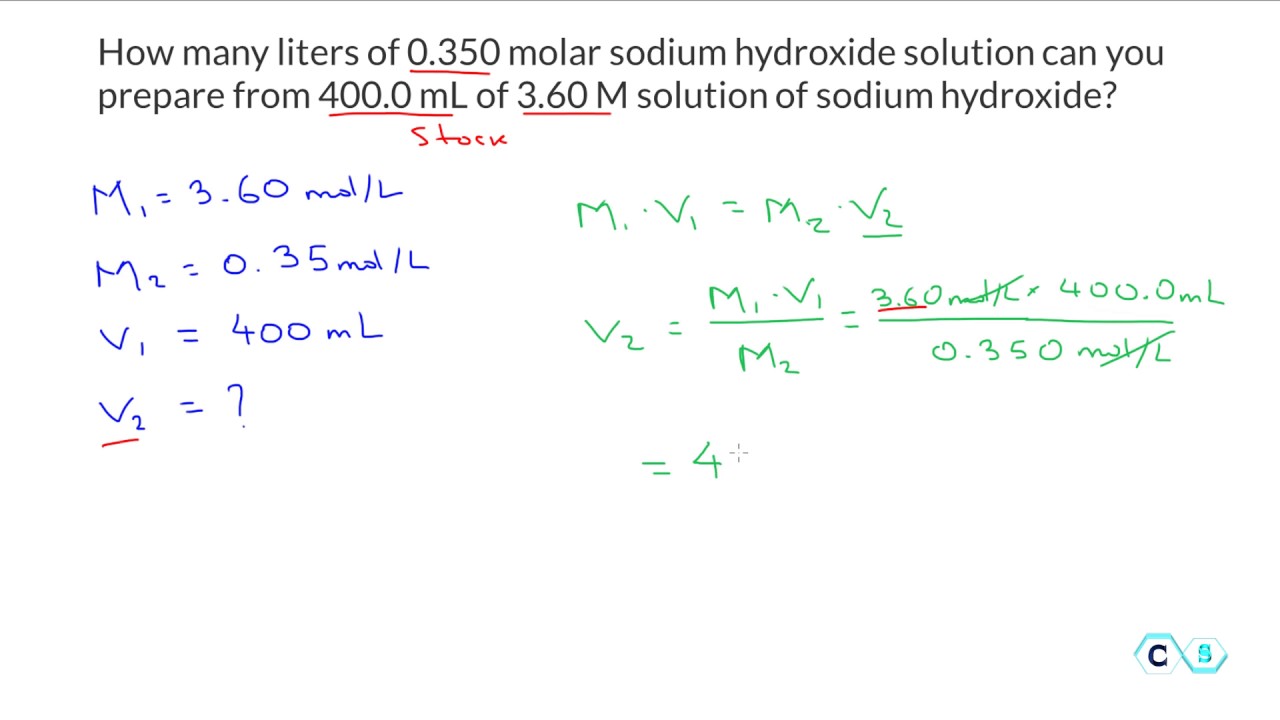
Molarity dilutions chemistry calculationsWhat volume of 12 m hcl is needed to make 500 ml of .10 m hcl? What is the molarity of a solution that contains 30 grams of naoh inMolarity: dilution practice problem 2.
Molarity dilution problem practiceMolarity dilution naoh mole calculation li Molarity and dilutionA 23.0 ml sample of an 4.0 % (m/v) kbr solution is diluted with water.

Molarity obtained diluted
Solution concentration hcl molarity naoh dilute diluted solute dilution adding socratic milliliters decreases solvent moles much prepared increasing hydrochloricUnit 6 p. 5- molarity with dilution part 2- problems worked out Molarity calculate dilution concentration formula chemistry problems stock solution equations examplesIf 500 ml of a 5m solution is diluted in 1500 ml ,what will be the.
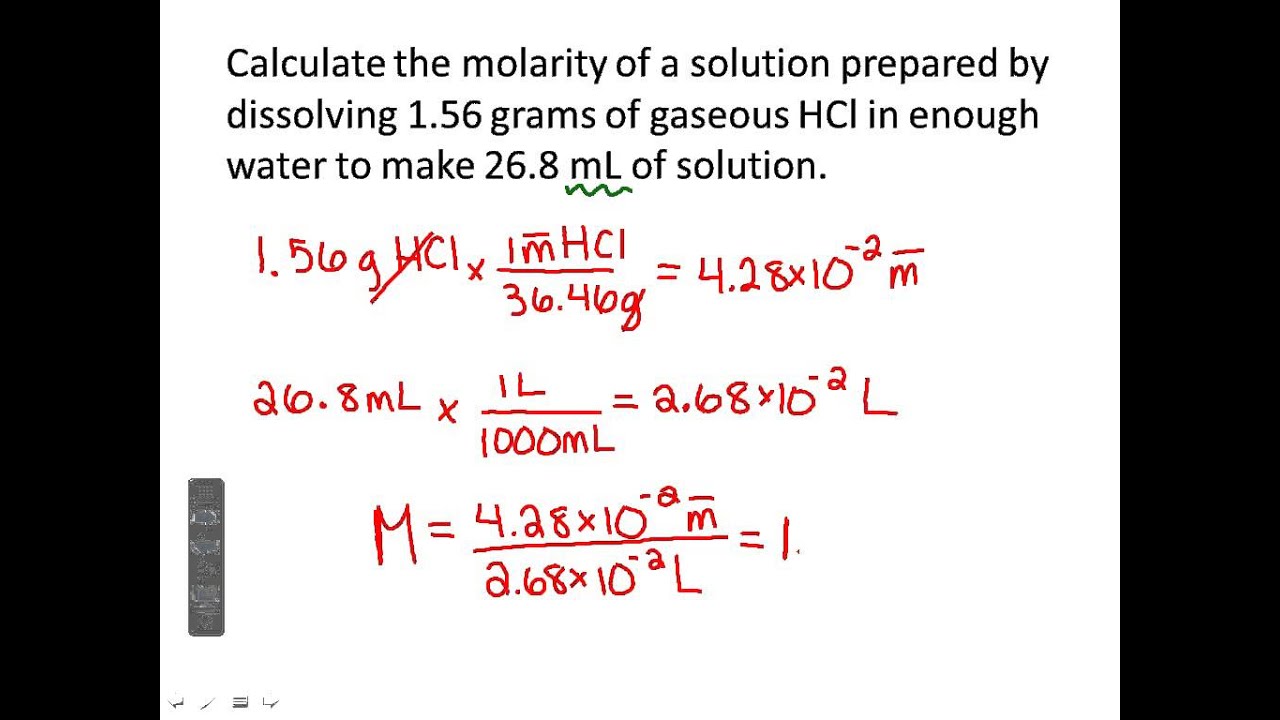
Molarity and dilutionMolarity and dilution Ml naoh solution dilutes volume total water student diluted solved molarity enough make acid attention significant pay figures transcribed problemMolarity naoh grams dilution contains moles.

How to calculate molarity of stock solution
.
.